Abstract
Introduction
Acute Myeloid Leukemia (AML) is a disease that affects predominantly older patients with a median age at diagnosis of 68 years. Elderly patients often have medical comorbidities and are ineligible for intensive induction chemotherapy. Recently, the FDA approved the combination of HMA/venetoclax for patients with newly diagnosed AML who are 75 years or older, or who have comorbidities precluding intensive induction chemotherapy. Through this retrospective analysis of patients treated at a metropolitan academic leukemia center and academic-affiliated community oncology practice network, our goal was to evaluate the safety and health care resource utilization of HMA/venetoclax treatment in a real-world experience.
Methods
We performed a retrospective analysis of patients treated for AML with HMA/venetoclax as standard-of-care at UCLA from 2018 to 2021. Patients treated with first-line HMA/venetoclax at UCLA community clinics (CC) and at UCLA main campus (academic centers, AC) and had a treatment record available in the electronic medical system were included. We specifically sought to collect safety parameters. Short-term efficacy outcomes were secondary endpoints.
Results
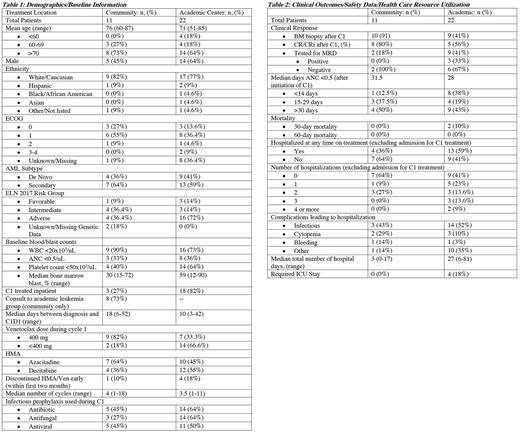
Thirty-three patients were eligible for analysis: eleven patients who received care in CC and 22 patients who were treated at AC. Baseline characteristics and treatment information are presented in Table 1. The average age was 76 years old for CC and 71 years old for AC. The proportion of patients older than 60 years old is 100% in CC, and 82% at AC. ECOG performance status was predominantly 0 and 1, irrespective of treatment location. Both groups have predominantly a secondary AML diagnosis (CC 64%, AC 59%). According to ELN 2017 criteria, 36.4% of CC had adverse risk disease, compared to 72% of AC patients. CC patients had median of 30% bone marrow blasts at diagnosis and AC had 59% blasts. Forty percent of CC patients and 64% of AC patients had baseline platelet counts <50x103/uL. Regarding treatment, eight out of 11 CC patients had consultation with a physician from the academic leukemia group. Twenty-seven percent of CC patients and 82% of AC patients were treated inpatient for cycle 1. Eighteen percent of CC patients and 66.6% AC patients received venetoclax at a starting dose lower than 400mg daily. Eighty-six percent of the dose reductions were due to concurrent azoles use. Finally, prophylactic antibiotics were used in 45% of CC patients compared to 64% of AC patients, and antifungal prophylaxis was used in 27% of CC patients compared to 64% of AC patients.
Safety data, health care resource utilization, and clinical outcome information are reported in Table 2. The percentage of patients with grade 4 neutropenia duration >30 days after cycle 1 was 50% in CC vs. 43% at AC. There were no deaths before 30 days at CC and there were 2 deaths (10%) in AC. Thirty- to sixty-day mortality was zero in both groups. Thirty-six percent of CC patients and 59% of AC required hospitalization (infectious related: 43% CC, 52% AC; cytopenia: 29% CC, 10% AC). The median number of hospital days was 3 (range, 0-17) in CC, and 27 (range, 6-81) in AC; and 0% of CC vs. 18% of AC patients required an ICU stay. Regarding clinical response, bone marrow biopsy was done after cycle 1 in 91% of CC vs. 41% of AC patients. Of these patients, CR/CRi rate was 80% in CC patients vs. 56% in AC patients.
Discussion
Our study suggests that AML treatment with HMA/venetoclax can be administered safely in real-world community setting. The high number of CC patients that had consultation with AC group reflects the integrative collaborative practice model at our urban academic affiliated oncology community practice. Patients treated in CC tend to be older, with more favorable AML, and better BM function reserve at baseline. The CR rate for patients treated in CC is comparable to previously published data. The high number of patients treated inpatient for cycle one at AC likely indicates AC carries a more acutely ill AML patient population compared to CC, which likely resulted in more health care resource utilization. It is interesting to observe that in the CC group, 29% of the complications leading to hospitalization are from cytopenia, suggesting that health care resource utilization could be further optimized with pre-emptive transfusion scheduling. Limitations of this analysis include that this is a small, single center, retrospective analysis.
Disclosures
Schiller:Janssen: Research Funding; AltruBio: Research Funding; Kite, a Gilead Company: Research Funding, Speakers Bureau; Stemline: Speakers Bureau; CTI: Research Funding; Ono Pharma: Honoraria; FujiFilm: Research Funding; Agios: Consultancy, Honoraria; Samus: Research Funding; Geron: Research Funding; Jazz: Consultancy; Cellectis: Research Funding; Regimmune: Research Funding; Pfizer: Research Funding; Astellas: Research Funding, Speakers Bureau; Constellation: Research Funding; Stemline: Research Funding; Amgen: Current equity holder in publicly-traded company, Honoraria; Celgene: Consultancy, Research Funding, Speakers Bureau; Deciphera: Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company; AbbVie: Research Funding, Speakers Bureau; Glycomimetics: Research Funding; AVM Biopharma: Research Funding; Actinium: Research Funding; Gilead: Research Funding; Genentech-Roche: Research Funding; Forma: Research Funding; AstraZeneca: Honoraria; Novartis: Honoraria, Other: Speaker fees, Research Funding; Actuate: Research Funding; Arog: Research Funding; Millennium: Research Funding; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; Gamida: Research Funding; Sellas: Research Funding; Medimmune: Research Funding; Trovagen: Research Funding; Cellerant: Research Funding; Cyclacel: Research Funding; Mateon: Research Funding; Deltafly: Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; Karyopharm: Research Funding, Speakers Bureau; Daiichi-Sankyo: Research Funding; PreCOG LLC: Research Funding; Onconova: Research Funding; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal